Sodium State At Room Temp

Pure sodium was first isolated by sir humphry davy in 1807 through the electrolysis of caustic soda naoh.
Sodium state at room temp. When pressure is controlled other pure elements may be found at room temperature. Sodium bromide is used in conjunction with chlorine as a disinfectant for hot tubs and swimming pools. Sodium is the sixth most common element on earth and makes up 2 6 of the earth s crust. This very soluble salt has been leached into the oceans over the lifetime of the planet but many salt beds or lakes are found where ancient seas have evaporated.
That state of matter of an element may be predicted based on its phase diagram. While temperature is an easily controlled factor manipulating pressure is another way to cause a phase change. Sodium bromide is used to prepare dense fluids used in oil wells. Sodium carbonate is an important commercial chemical.
Their alloys however are not. Since sodium can ignite on contact with water it must be stored in a moisture free environment. The commercially available 78 k 22 na alloy stays liquid at temperatures as low as 12 6 o c 9 3 o f. Sodium and its close periodic table neighbor potassium are solids at room temperature.
Although sodium is the sixth most abundant element on earth and comprises about 2 6 of the earth s crust it is a very reactive element and is never found free in nature. However this is a single dose value. Though rarely found in its elemental form as pure sodium metal because of its high reactivity it is a soft shiny metal that can be cut with a knife. It s crucial for blood pressure control and.
Sodium is a solid at room temperature. Sodium carbonate is a white crystalline powder or grayish white lumps. An example is the halogen element chlorine. It is also known as washing soda or soda ash.
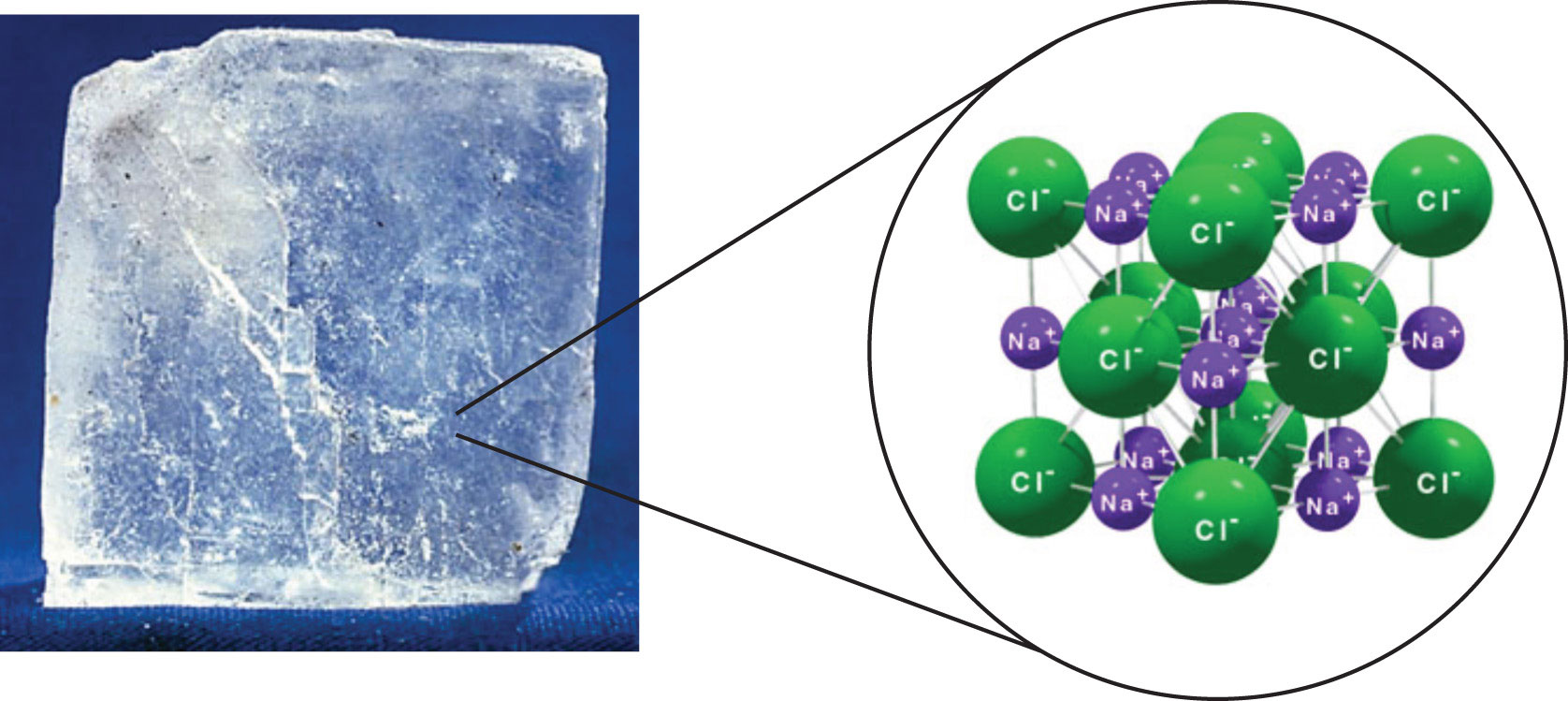
It is silver grey with brown touch. The most common compound is sodium chloride. Sodium is by far the most commercially important alkali metal. It is odorless and tasteless.
It is soluble in water. Other than table salt nacl sodium shows up in baking soda nahco 3 sodium peroxide na 2 o 2 and borax or sodium borate na 2 b 4 o7 10h 2 o. Nak alloys containing 10 to 60 percent of sodium by weight are liquids at room temperature. It conducts heat and electricity easily and exhibits the photoelectric effect emission of electrons when exposed to light to a marked degree.
Lighter than water sodium can be cut with a knife at room temperature but is brittle at low temperatures.