Sodium Hydroxide At Room Temperature

When dissolved in water or neutralized with acid it liberates substantial heat which may be sufficient to ignite combustible materials.
Sodium hydroxide at room temperature. Sodium hydroxide is an a compound. At room temperature sodium hydroxide is a white crystalline odorless solid that absorbs moisture from the air. As such it has a formula rather than a symbol. April 5 1999 vol.
It reacts with moisture from the air and may generate heat as it dissolves. Corrosion in caustic solutions. The table below provides information on the variation of solubility of different substances mostly inorganic compounds in water with temperature at one atmosphere pressure units of solubility are given in grams per 100 millilitres of water g 100 ml unless shown otherwise. The viscosity of aqueous naoh as with any liquid chemical is inversely related to its service temperature i e its viscosity.
At room temperature sodium hydroxide is a white crystalline odorless solid that absorbs moisture from the air. When dissolved in water or neutralized with acid it liberates substantial heat which may be sufficient to ignite combustible materials. It is a manufactured substance. The table was taken from perry s chemical engineers handbook by robert h.
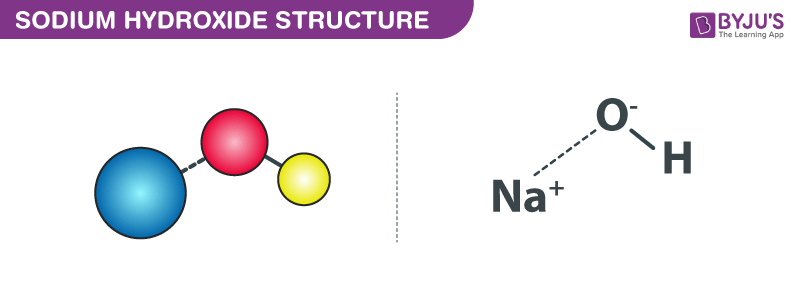
Sodium hydroxide is the main ingredient in household products such as liquid drain cleaners. The formula is naoh s. The merck index an encyclopedia of chemicals drugs and biologicals. Making sodium carbonate from sodium bicarbonate.
The table below gives the density kg l and the corresponding concentration weight of sodium hydroxide in water at different temperatures in degrees centigrade c. Sodium hydroxide is an alkaline corrosive. It is a manufactured substance. Triclinic leaflets becomes anhydrous at 38 c or in desiccator over potassium hydroxide at room temperature sodium chlorite trihydrate o neil m j.
Sodium hydroxide is very corrosive. 1 corrosion by caustic sodium or potassium hydroxide at all concentrations is easily handled at room temperature with a variety of metals and alloys including carbon steels. The state of sodium hydroxide is solid at a room temperature. At room temperature pure sodium hydroxide naoh is a white odorless solid.
Concentrated 50 aqueous solutions of sodium hydroxide have a characteristic viscosity 78 mpa s that is much greater than that of water 1 0 mpa s and near that of olive oil 85 mpa s at room temperature. The decomposition of anhydrous sodium carbonate into sodium oxide and carbon dioxide occurs slowly at room temperature and proceeds to completion at 851 c 1124 k.