Sodium Carbonate State At Room Temperature

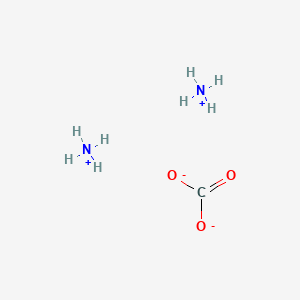
Sodium calcium carbonate an equimolar double salt na 2 co 3 caco 3 never described in the literature forms by a novel solid state reaction when intimately mixed na 2 co 3 and caco 3 powders are heated.
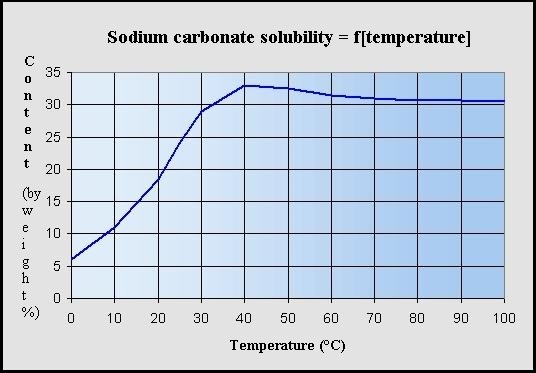
Sodium carbonate state at room temperature. Historically it was extracted from the ashes of plants growing in sodium rich soils. It is a solid at room temperature since it is ionic. Density g cm 3 density is the mass of a substance that would fill 1 cm 3 at room temperature. Soda ash also known as sodium carbonate na2co3 is an alkali chemical refined from the mineral trona or naturally occurring sodium carbonate bearing brines the soda ash from both is referred to as natural soda ash or manufactured from one of several chemical processes the soda ash from this process is referred to as synthetic soda ash.
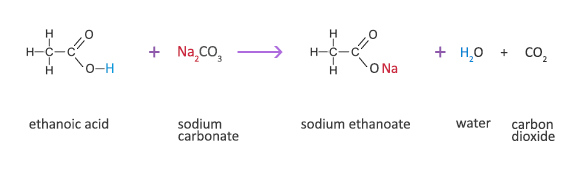
Making sodium carbonate from sodium bicarbonate. The elements in sodium bicarbonate are. Sodium bicarbonate iupac name. The temperature at which the liquid gas phase change occurs.
Sodium carbonate has long been known to be a good co 2 absorber vericella said but it s slower than amine solutions. The thermal synthesis and the properties of this compound are evaluated by thermal analysis using an instrument yielding simultaneous dta tg and ega data. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula naoh. Sodium na hydrogen h carbon c and oxygen o.
The decomposition of anhydrous sodium carbonate into sodium oxide and carbon dioxide occurs slowly at room temperature and proceeds to completion at 851 c 1124 k. With encapsulation however the surface area for absorption increases to. It is a white solid ionic compound consisting of sodium cations na and hydroxide anions oh. Nahco3 sodium bicarbonate is a compound.
All forms are white water soluble salts that yield moderately alkaline solutions in water. Sodium bicarbonate also called sodium hydrogen carbonate or bicarbonate of soda nahco 3 is a source of carbon dioxide and so is used as an ingredient in baking powders in effervescent salts and beverages and as the main constituent of dry chemical fire extinguishers. Relative atomic mass the mass of an atom relative to that of. Its slight alkalinity makes it useful in treating gastric or urinary.
Sublimation the transition of a substance directly from the solid to the gas phase without passing through a liquid phase.