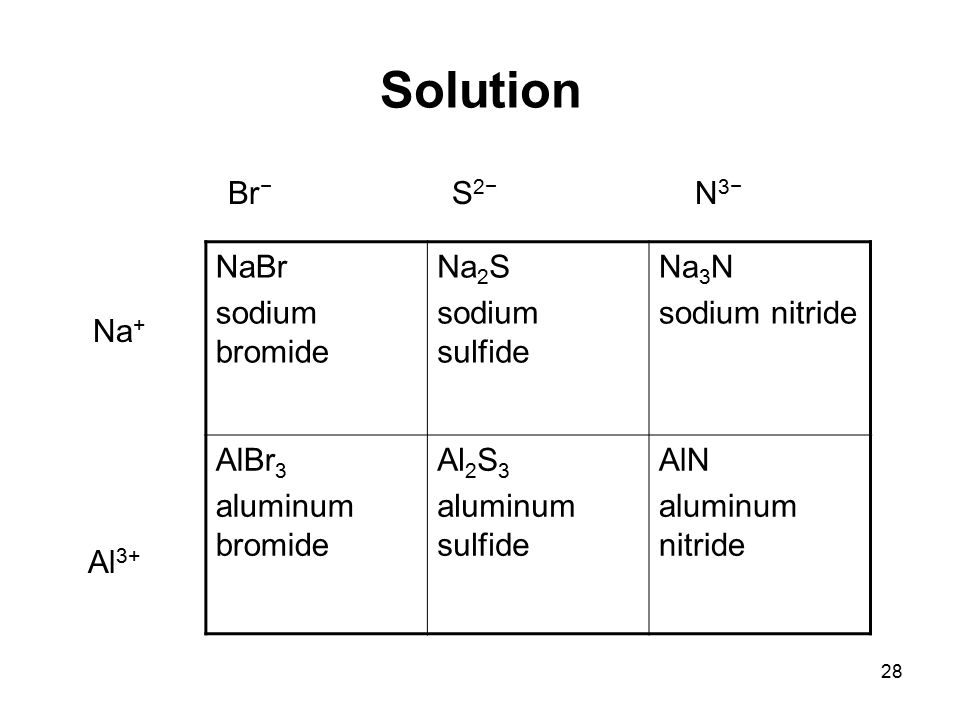
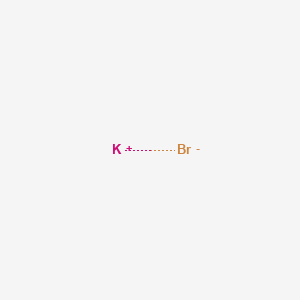Sodium Bromide At Room Temperature
Nabr has a very low toxicity with an oral ld 50 estimated at 3 5 g kg for rats.
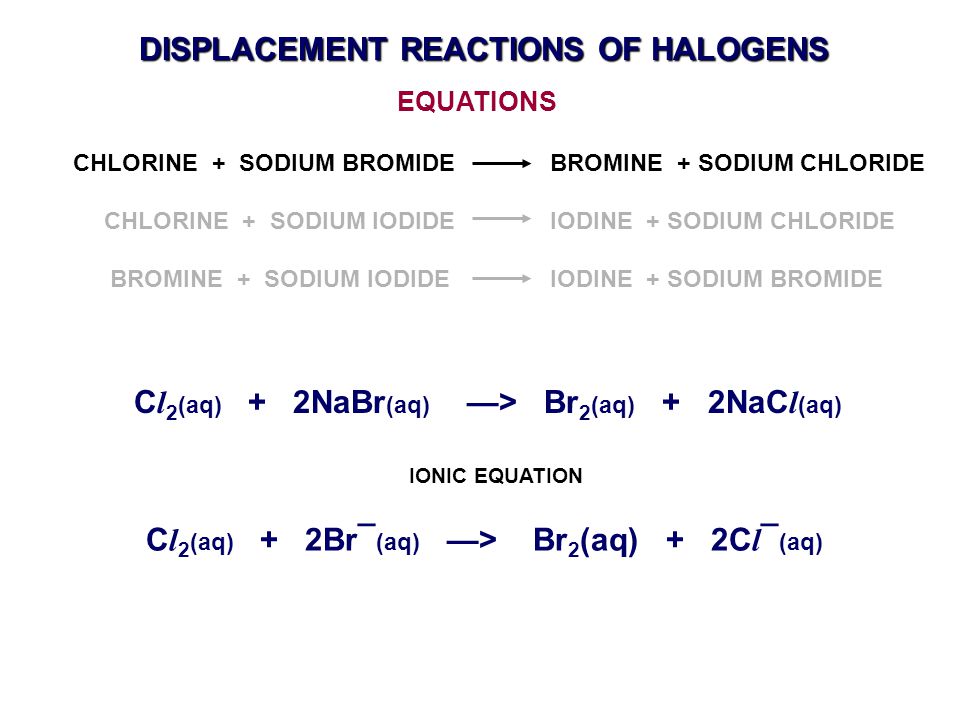
Sodium bromide at room temperature. It was used in medicine as a hypnotic. Sodium bromide is a white solid. The solution is known as bromine water. Sodium bromide is used in conjunction with chlorine as a disinfectant for hot tubs and swimming pools.
1 ii what is the state of sodium bromide at room temperature. Bromide is stable for more than 1 year at room or refrigerator temperature in glass plastic clear or brown containers when in solution with distilled water. Sodium is by far the most commercially important alkali metal. Lighter than water sodium can be cut with a knife at room temperature but is brittle at low temperatures.
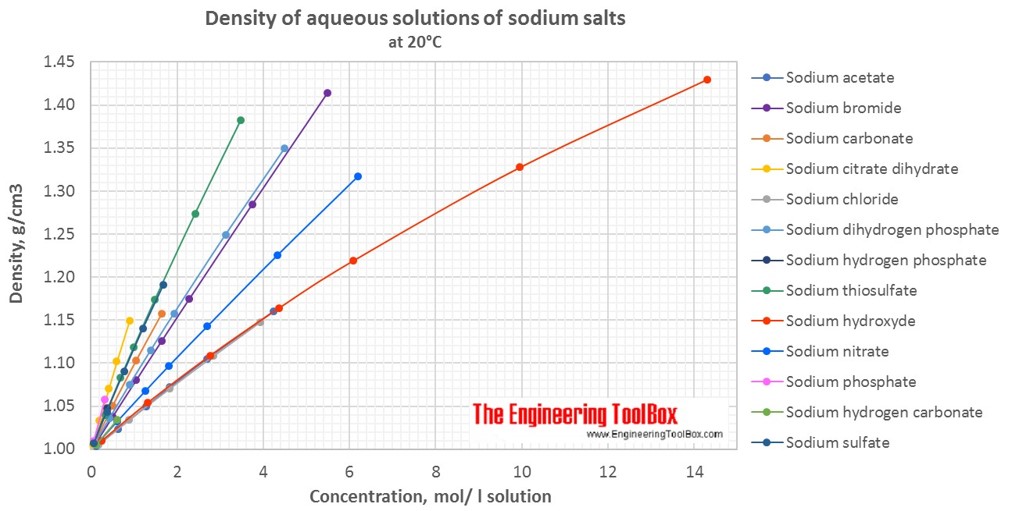
The table below provides information on the variation of solubility of different substances mostly inorganic compounds in water with temperature at one atmosphere pressure units of solubility are given in grams per 100 millilitres of water g 100 ml unless shown otherwise. D the diagram below shows the arrangement of the particles in sodium bromide at room temperature. The boiling point and hydrogen bromide concentration can be partially controlled by varying the pressure during distillation. About 3 41 grams 0 12 ounce of bromine dissolve in 100 millilitres 0 1 quart of water at room temperature.
Constant boiling hydrobromic acid distills at 124 3 c at atmospheric pressure and contains 47 63 wt hydrogen bromide. Like chlorine water it is a good oxidizing agent and it is more useful because it does not decompose so readily. Use the information in the diagram. From water of room temperature sodium bromide crystallizes with 2h2o in the form of colorless crystals.
The dose of sodium bromide should be reduced by 15 compared to the potassium salt to account for the higher bromide content per gram kbr 67 bromide nabr 78 bromide. When it is heated very strongly in air it makes bromine gas. The substances are listed in alphabetical order. It also reacts with chlorine to make liquid bromine.
Sodium bromide is used to prepare dense fluids used in oil wells. It conducts heat and electricity easily and exhibits the photoelectric effect emission of electrons when exposed to light to a marked degree. However this is a single dose value. These solutions are produced industrially by dissolution of hydrogen bromide in water.
It dissolves easily in water.