Sodium Bicarbonate State At Room Temperature

It is a solid at room temperature since it is ionic.
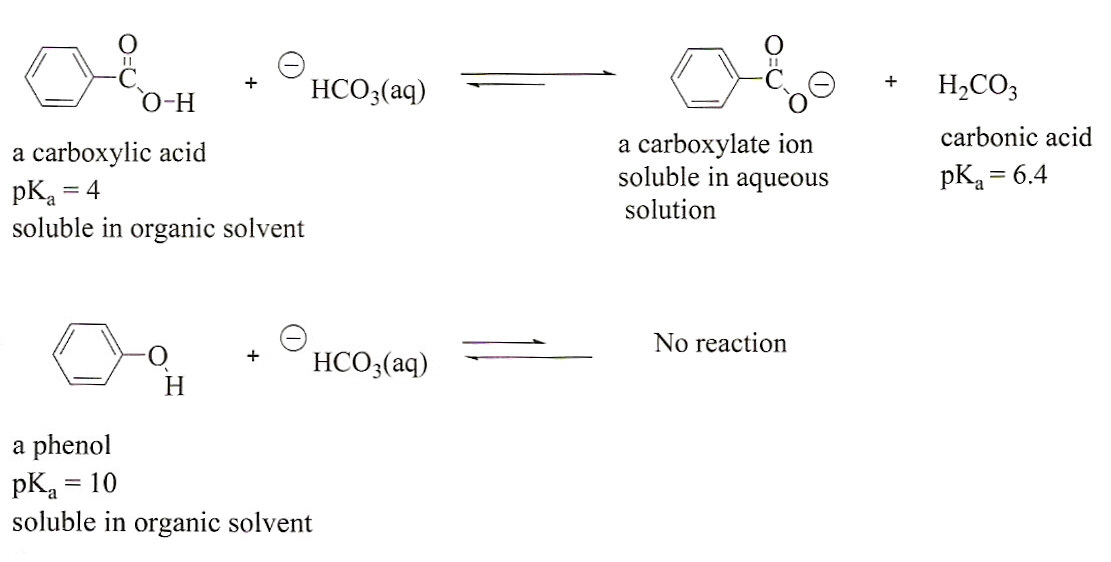
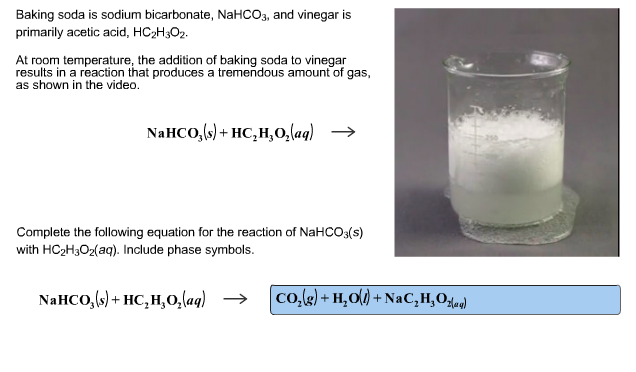
Sodium bicarbonate state at room temperature. Store at room temperature away from moisture and heat. Mechanism of action of sodium bicarbonate sodium bicarbonate in water dissociates to sodium na and bicarbonate hco3 ions. Sodium bicarbonate appears as odorless white crystalline powder or lumps. Sodium bicarbonate also called sodium hydrogen carbonate or bicarbonate of soda nahco 3 is a source of carbon dioxide and so is used as an ingredient in baking powders in effervescent salts and beverages and as the main constituent of dry chemical fire extinguishers.
Its slight alkalinity makes it useful in treating gastric or urinary. As the temperature increases to the boiling point of water 100 celcius the reaction goes to completion with the decomposition of all the sodium bicarbonate. Ph of freshly prepared 0 1 molar aqueous solution. Moderately soluble in water weak hydrolysis on the anion.
Sodium bicarbonate iupac name. Slightly alkaline bitter taste. Sodium na hydrogen h carbon c and oxygen o. Baking soda salt bulriha nahkolit.
The elements in sodium bicarbonate are. Properties of sodium bicarbonate nahco3. Call your doctor if your symptoms do not improve or if they get worse while using sodium bicarbonate. Sodium hydrogen carbonate commonly known as baking soda especially in north america and new zealand or bicarbonate of soda is a chemical compound with the formula nahco 3 it is a salt composed of a sodium cation na and a bicarbonate anion hco 3 sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder.
Nahco3 sodium bicarbonate is a compound. Sodium bicarbonate about sodium bicarbonate sodium compound systemic antacid alkalinizer. Sodium na is the principal cation of the extracellular fluid and it maintains fluid and electrolyte disturbances. In the wet state begins to decompose at room temperature.
It is a solid at room temperature since it is ionic. The elements in sodium bicarbonate are. White at low heat decomposes. Follow al directions on the product label.
Crystalline hydrates do not form. Ph of saturated solution. Nahco3 sodium bicarbonate is a compound. Sodium na hydrogen h carbon c and oxygen o.




































:max_bytes(150000):strip_icc()/__opt__aboutcom__coeus__resources__content_migration__treehugger__images__2016__09__baking_soda-a81d7b8411b84674b6ef4a19f3776d4b.jpg)




