Sodium At Room Temperature State Of Matter

No matter what state matter is in it will always have the same mass assuming it.
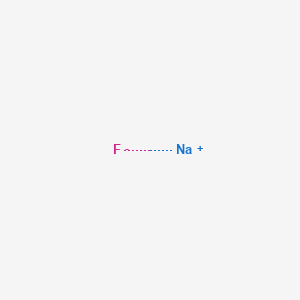
Sodium at room temperature state of matter. Though rarely found in its elemental form as pure sodium metal because of its high reactivity it is a soft shiny metal that can be cut with a knife. When a certain temperature threshold unique to each substance in the universe is crossed a phase change will result changing the state of the matter. The elements in sodium bicarbonate are. The state a given substance exhibits is also a physical property.
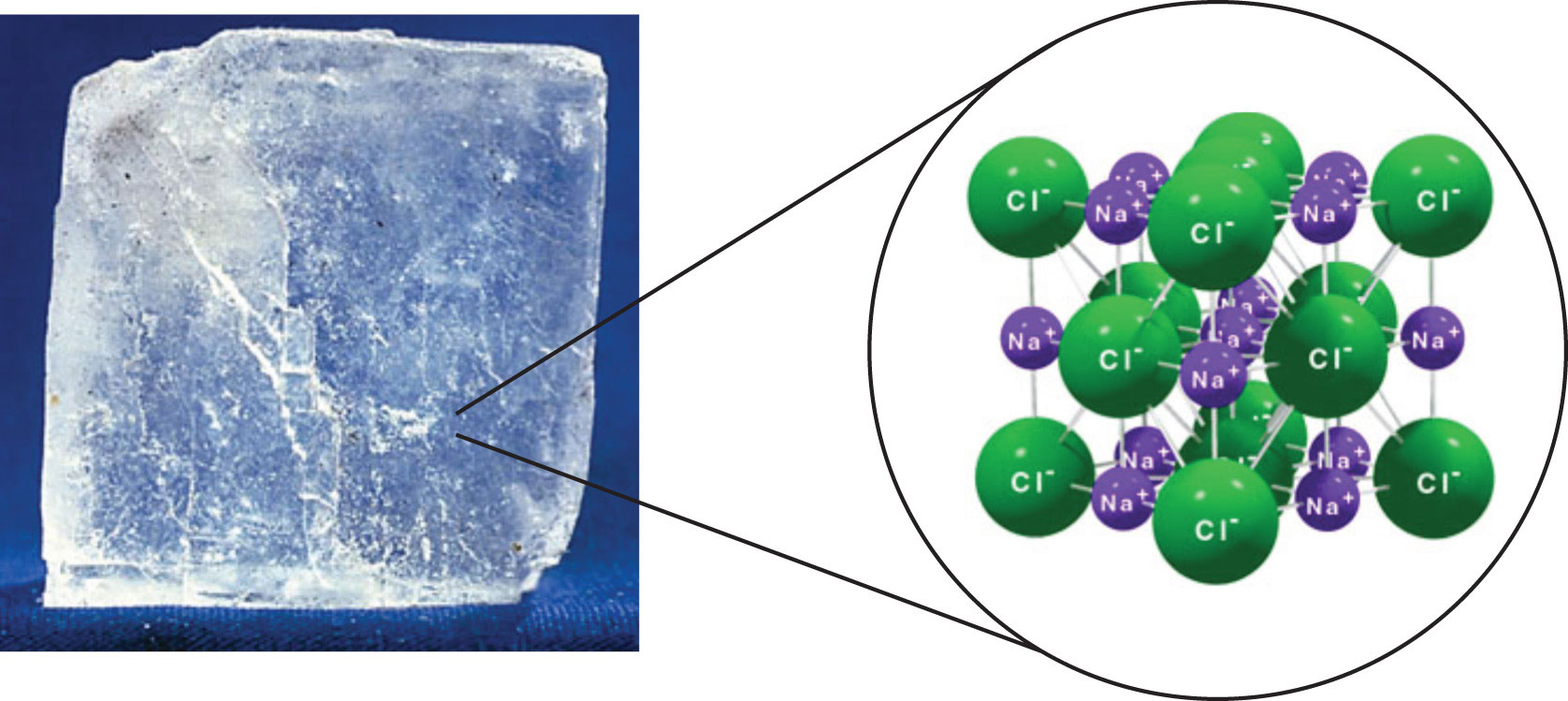
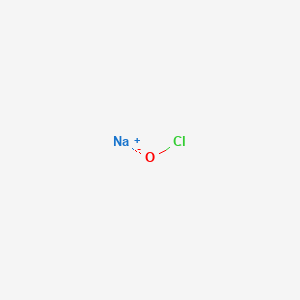
Sodium chloride or nacl is a solid at room temperature 70 degrees fahrenheit. Most metals exist as solids at room temperature. Some substances exist as gases at room temperature oxygen and carbon dioxide while others like water and mercury metal exist as liquids. Sodium is by far the most commercially important alkali metal.
Sodiums state at room temperature. Sodium is a solid at room temperature. Though rarely found in its elemental form as pure sodium metal because of its high reactivity it is a soft shiny metal that can be cut with a knife. Asked by wiki user.
Matter can exist in a solid liquid or gaseous state and the state a substance is in can be largely determined by its temperature. Lighter than water sodium can be cut with a knife at room temperature but is brittle at low temperatures. What is sodium s state of matter. It is a solid at room temperature since it is ionic.
The temperature at which the liquid gas phase change occurs. Sodium na hydrogen h carbon c and oxygen o. Sublimation the transition of a substance directly from the solid to the gas phase without passing through a liquid phase. Density g cm 3 density is the mass of a substance that would fill 1 cm 3 at room temperature.
Nahco3 sodium bicarbonate is a compound. It conducts heat and electricity easily and exhibits the photoelectric effect emission of electrons when exposed to light to a marked degree. It is also known as table salt outside of the scientific nomenclature. Relative atomic mass the mass of an atom relative to that of.